Which of the Following Is Chemically Inert Unreactive
They are present on the left side of the periodic table. Oxygen atomic number 8 carbon atomic number 6 sodium atomic number 11 neon atomic number 10 false Chemical properties are determined primarily by neutrons.
Compounds with fluorine such as KrF2 KrF4 Why are noble gasses chemically inert.
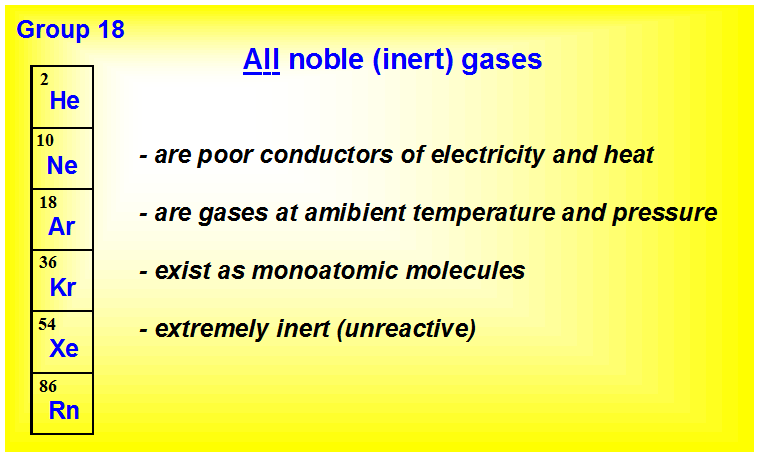
. Which of the following is chemically inert unreactive. Which of the following is chemically inert unreactive. Most Group 8 or 18 elements that appear in the last column of the periodic table Helium Neon Argon Krypton Xenon and Radon are classified as inert or unreactive.
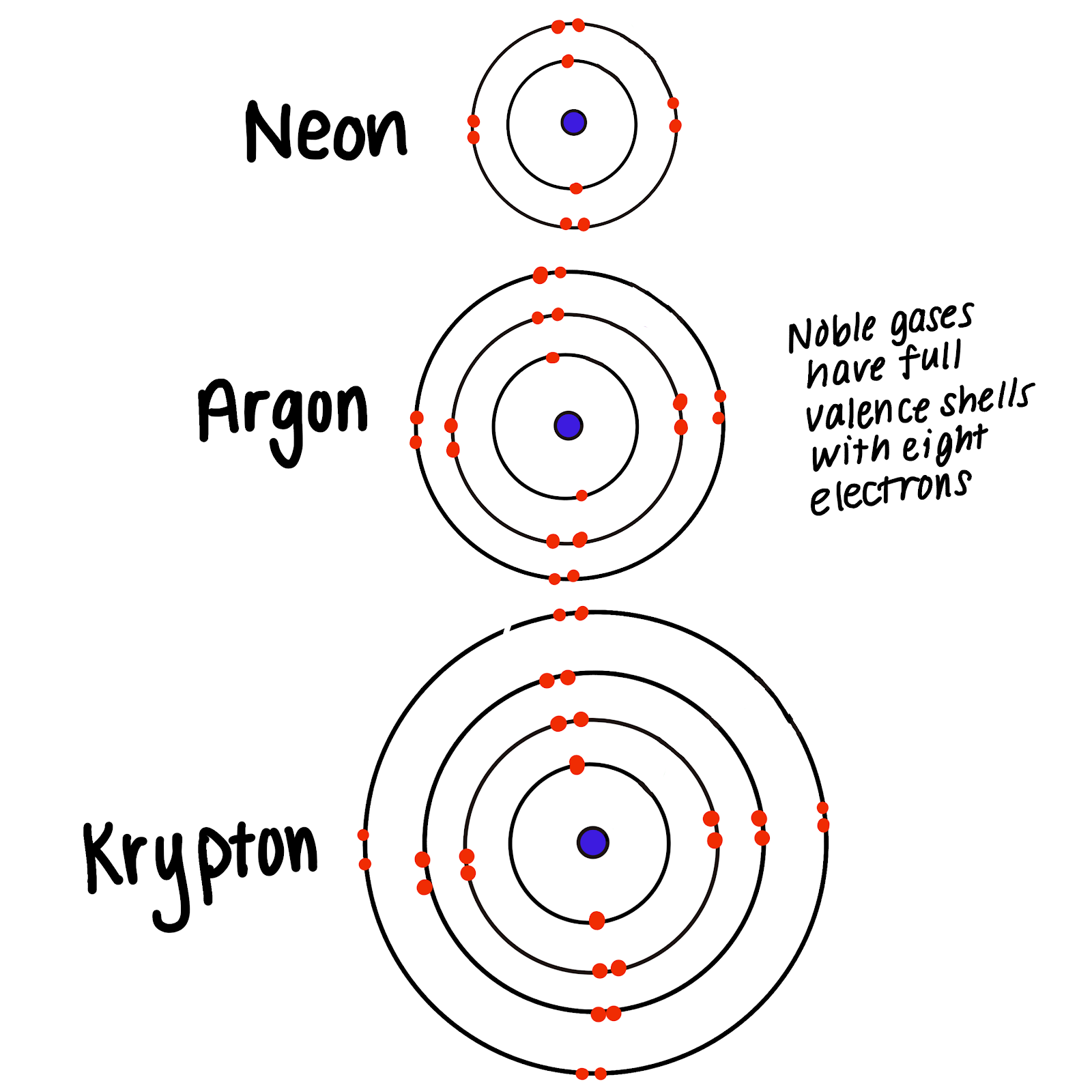
So it is chemically extremely unreactive. Helium He neon Ne argon Ar krypton Kr xenon Xe and radon Rn. Noble gases have a full valence shell.
These are elements that does not react chemically elements found in group 8 of the periodic table are enlisted here such as the xenon argon neon krypton Radon. Noble gases are present in the farthermost right side of the periodic table. What process do mrna and trna work together to complete.
These elements are stable in their naturally occurring form gaseous form and they are called inert gases. However when nitrogen bonds do break the resulting products are often highly reactive. The name comes from the fact that these elements are virtually unreactive towards other elements or compounds.
Synonyms crossword answers and other related words for UNREACTIVE inert We hope that the following list of synonyms for the word inert will help you to finish your crossword today. Elements are divided into various categories. Apathetic 5 Of sluggish nature 5 Like atoms with full outer shells 5 Unreactive 5 Sluggish by nature 5 Like xenon and krypton 5 Like a noble gas 5 Physically inactive 5 Disinclined to move 5 Not reacting 5 Unable to react 5 Like propellants in pesticides 5.
Chemically unreactive 5 INERT. Survey Did this page answer your question. Nitrogen is a chemical element with an atomic number of 7 it has seven protons in its nucleus.
Which of the following is chemically inert unreactive. A sodium atomic number 11 B oxygen atomic number 8 C carbon atomic number 6 D neon. Inanimate while unreactive is chemistry not reactive.
A carbon atomic number 6 B neon atomic number 10 C oxygen atomic number 8 D sodium atomic number 11. 3 on a question Which of the following is chemically inert unreactive. The Chemistry of Life PRE-LAB QUESTIONS 1.
They are inert in nature or are unreactive. Most Group 8 or 18 elements that appear in the last column of the periodic table Helium Neon Argon Krypton Xenon and Radon are classified as inert or unreactive. Biology 21062019 2020 jaylahlove77.
Contents 1 Noble gas 2 Inert gas 21 Main uses 3 References Noble gas. Weve arranged the synonyms in length order so that they are easier to find. 3 Get Other questions on the subject.
Chemically inactive 5 Lifeless. Which of the following is chemically inert unreactive. In chemistry the term chemically inert is used to describe a substance that is not chemically reactive.
Helium is chemically extremely unreactive. Helium has completely filled outer shell. Reactivity of an element is defined as the tendency of an element to loose or gain electrons.
Group 8A or VIIIA of the periodic table are the noble gases or inert gases. Its electronic configuration is 1s2and it is very stable state configuration. However this rule is justified for elements of second row in the periodic table which their outermost - shell capacity is 8 electrons.
Helium is unreactive and is chemically inert. They were long believed to be totally unreactive. The strong triple-bond between the atoms in molecular nitrogen makes this compound difficult to break apart and thus nearly inert.
Place in order for an atom to. As a noun inert is chemistry a substance that does not react chemically. Which of the following is chemically inert unreactive.
Chemically inactive 5 Lifeless. As adjectives the difference between inert and unreactive is that inert is unable to move or act. Rare earth metals are present in group 3 from the left.
The eight- electrons stability of an atom is stemmed from the stability of the noble gases or the elder name inert gases which had long been known as unreactive or noble. View Lab 5docx from BIO 105 at Kishwaukee College. Metals are the elements which loose electrons.
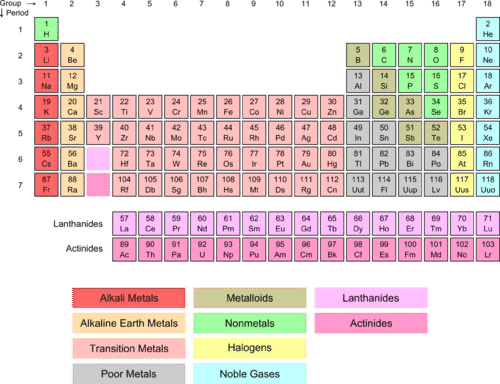
Any of the gaseous elements helium neon argon krypton xenon and radon occupying Group 0 18 of the periodic table. Transition metals are present in the center of the periodic table from group 3 to group 12. Not at all Slightly Kinda Very much Completely.
Biology 22062019 1330 sabinaschaaf10. The chemically inert element is Neon. What is any method of measuring the age of an object or.
They are present in group 18. Nitrogen fixation is a natural process by which inert or unreactive forms of nitrogen are transformed.
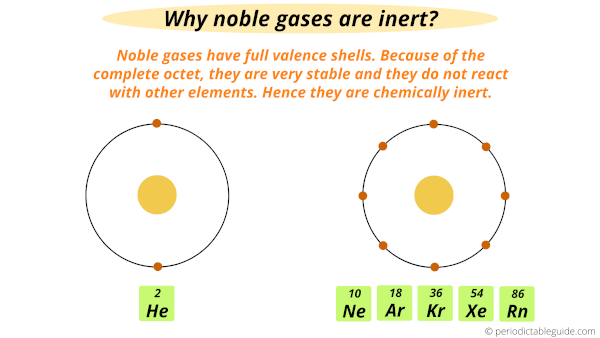
Where Are Noble Gases Located On The Periodic Table
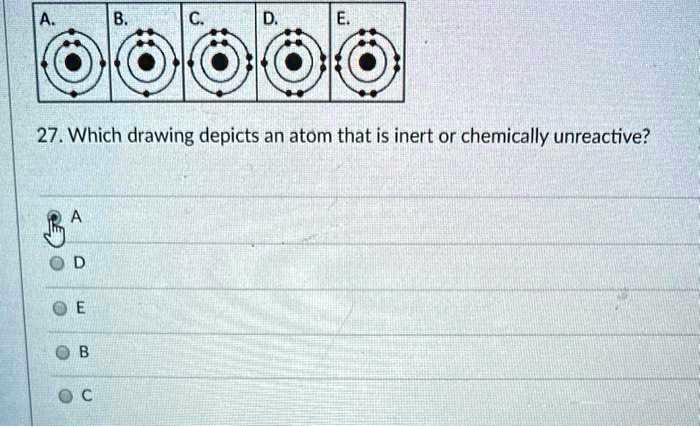
Solved 27 Which Drawing Depicts An Atom That Is Inert Or Chemically Unreactive
Why Don T Noble Gases Bond Video Lesson Transcript Study Com
What Does Inert Mean In Chemistry Quora
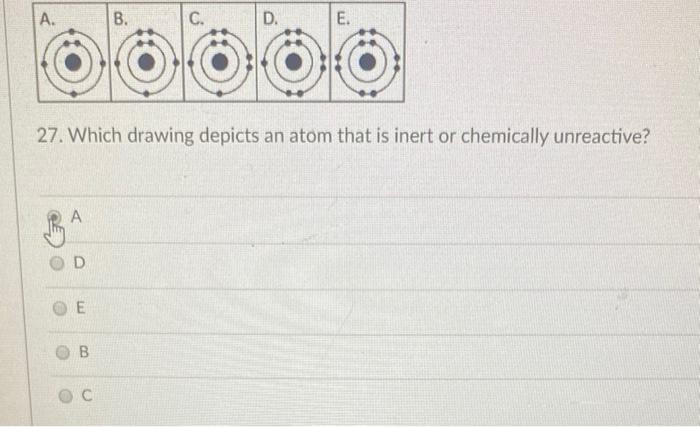
Solved A B C D E 27 Which Drawing Depicts An Atom That Chegg Com

Comments
Post a Comment